Chemistry is one of the widest subjects in existence. When you think of chemistry, the first thing that comes to your mind is the term element. The term element is used widely in the subject to refer to the basic components of matter, compounds, and everything that exists in the world. There are several types of elements in Chemistry. Each element has its characteristics that differ from the rest. Thus, these elements are organized in a popular structure in chemistry known as the periodic table. There are two models of the table; periodic table alphabetical and the latter.
How is the periodic table organized?
The periodic table consists of all elements in Chemistry. The elements are organized in informative cells and are arranged in the order of their atomic number and chemical properties. They are arranged in such a way that these properties increase from one element to the other.
For instance, the elements would be organized in order of increasing atomic number. Elements on a periodic table that belong to the same group are those that share the same number of valence electrons. These elements also behave the same when it comes to bonding with other elements and chemical reactions in the process. The cells in which the elements are contained in the periodic table detail important information about each element.
The periodic table consists of rows and columns. On the rows, information on the periods is provided while the columns are organized in groups. Elements that belong to the same group are those that share the same electron affinity, and they react in the same manner when bonding with their counterparts.
Information found in the cells of a periodic table
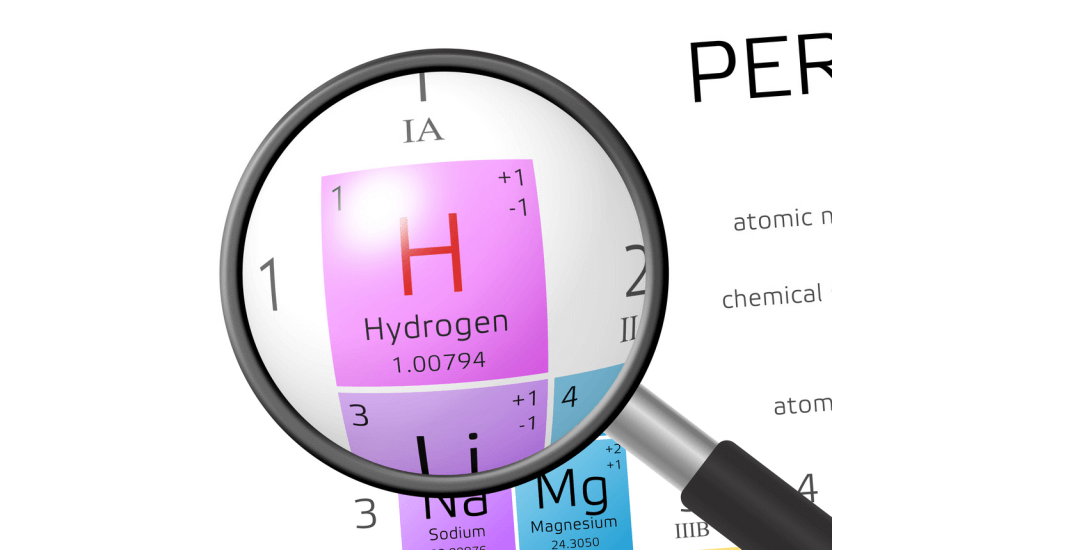
The first thing you will learn about an element from the cells of a periodic table is the name of the element. The name is accompanied by its symbol. However, not all modes of periodic tables have two features. Some show the symbols and not the name. However, most of them consist of the name and symbol because most people do not know how to derive the name from the symbol.
The symbol of an element refers to the abbreviation of the name either in Latin or English. Most of the element symbols, as seen on the periodic table, are a single letter or two and nothing more.
On the periodic table, you also get to see the atomic number of each element since it is used in their organization. The atomic number refers to the number of protons contained in an atom of the element in question.
You also get to find the relative atomic mass on the periodic table. The relative atomic mass refers to the average weight of the isotopes in an element. This information is used to calculate the molar mass of a single element or a compound.
Conclusion
While there are different types of periodic tables provided by different institutions, the basics of the table are the same in each. The purpose of the table and the elements contained in it are also the same. Some may contain a portion of the elements, while more complex ones consist of all the elements in existence.